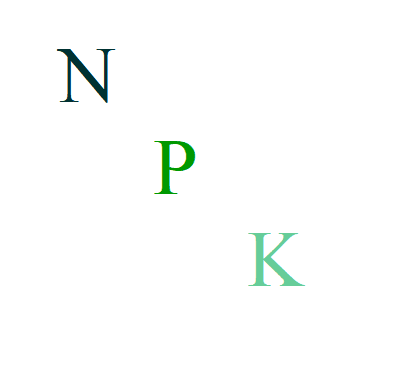
What is an NPK Fertilizer?
Once bitten by the gardening bug, inevitably you will come across the term NPK fertilizer. We’ll demystify what NPK signifies, outline its advantages, and provide practical examples of its usage.
The term NPK fertilizer may sound cryptic, leaving you puzzled about its meaning. What do N, P, and K stand for? The answer to this question is just a paragraph away. Subsequently, we’ll introduce some common NPK fertilizers, delving into their pros and cons after elucidating the distinctions between organic, mineral, and organic-mineral NPK fertilizers.
What exactly are NPK fertilizers?
Let’s start with the three letters: N stands for Nitrogen, P for Phosphorus, and K for Potassium. These elements are classified as macronutrients, essential in substantial quantities for the growth and healthy development of plants. An NPK fertilizer is a fertilizer containing at least these three essential nutrients, with some formulations including additional nutrients such as Ca (Calcium), Mg (Magnesium), and S (Sulfur), also considered macronutrients.
Below, we provide insights into the functions Nitrogen (N), Phosphorus (P), and Potassium (K) serve for plants:
Nitrogen (N):
Primarily utilized by plants for building proteins, DNA, and chlorophyll—the green pigment crucial for photosynthesis. Nitrogen is often referred to as the “engine of vegetative growth.” A common garden fertilizer often has nitrogen as the largest ingredient. However, a good fertilizer for cacti and succulents requires significantly less nitrogen or a balanced NPK composition.
Nitrogen is required in large quantities and is found in all proteins, crucial for binding atmospheric carbon dioxide to carbohydrates during photosynthesis. Abundant nitrogen fertilization results in increased leaf mass, with nitrogen-demanding crops like lettuce and other leafy greens benefiting greatly. Flowering is often delayed when the plant prioritizes leaf growth. Nitrogen deficiency leads to small plants with yellow-green, often undersized leaves.
Excessive nitrogen causes dark green, puckered leaves and makes the plant more susceptible to diseases and insect attacks. Fertilizers high in nitrogen include chicken manure and urine. Urine contains approximately 5g of nitrogen, 1g of phosphorus, and 2 grams of potassium per litre. Diluting urine with 10–15 parts water achieves similar concentrations as pre-mixed liquid fertilizers, a cost-effective and resource-saving option. For an equivalent strength with chicken manure, mix half a deciliter of fresh chicken manure in 10 litres of water.
It is typically recommended to dilute nettle water with about 10 parts water, but the nutrient content in such a mixture is considerably lower than the doses recommended for liquid fertilizers (see table). Nettle water that has been left to ferment for a few weeks has a nitrogen content of approximately 0.3–0.4 grams per litre.
Phosphorus (P):
Found in proteins (specifically enzymes) and involved in DNA structure, Phosphorus is a key component of ATP (Adenosine Triphosphate), the energy currency of all cells. Additionally, Phosphorus stimulates flower and fruit formation, particularly relevant for avid gardeners.
Phosphorus is the nutrient that, next to nitrogen, most commonly experiences a deficiency in natural ecosystems, although it is uncommon in our gardens. Phosphorus is crucial for the plant’s energy balance and is present in cell nuclei and cell membranes. Deficiency leads to poor growth, red or bronze-colored leaves, and inadequate flowering.
Phosphorus is abundant in bone residues and fish remains, making old cultivated soils often rich in phosphorus. Archaeologists have utilized this to identify ancient settlements during archaeological excavations. Sandy soils are often phosphorus-poor and require fertilization, while phosphorus in calcareous soils tightly binds to lime, rendering fertilization less effective. However, plants in these soils have adapted to access bound phosphorus.
Chicken and pig manure are rich in phosphorus, whereas horse and cow manure are less so. Bone meal is an excellent but relatively expensive way to increase phosphorus levels in the soil.
Potassium (K):
Unlike Nitrogen and Phosphorus, Potassium is not directly involved in building new cell structures. Instead, plants store Potassium in their vacuole, the central water-filled space in each cell. This high concentration aids the cell in better water absorption and retention. Potassium also plays a role in regulating the plant’s overall water balance. Moreover, it supports enzyme activity, influencing the plant’s metabolism, contributing to the formation of robust cell walls, enhancing resistance to fungi, and deterring sucking insects.
In the wild, cacti and succulents live in arid landscapes with a natural lack of nitrogen and have over time adjusted to these conditions and require them to be happy plants.
Potassium always occurs in a soluble form in plants and is not part of any organic molecules. Potassium is necessary to regulate water and salt balance in plants. Potassium deficiency often results in dry leaf edges. Swine and cattle manure contains a significant amount of potassium, but the easiest way to supply potassium is through wood ash, which can contain several percent potassium. A few handfuls of wood ash per square meter are usually sufficient to meet the potassium needs of most kitchen plants.
Other nutrients that plants require include magnesium (which is part of chlorophyll) and calcium (which is part of cell walls), but deficiencies in these elements are rare. Additionally, various micronutrients are necessary, but when using organic fertilizers, it’s seldom necessary to supplement them.
The NPK Composition
If you flip the packaging of a fertilizer, you’ll encounter a set of numbers, usually in a square box. It could say, for example: “10 – 5 – 6.” These numbers indicate the percentage of nitrogen compounds, phosphorus compounds, and potassium compounds in the fertilizer. It’s essential to note that these nutrients are presented as chemical compounds in the fertilizer, not in elemental form.
Types of NPK Fertilizers:
Within the variety of NPK fertilizers, distinctions can be made between mineral, organic, and organic-mineral fertilizers. Let’s briefly explore each type:
Mineral Fertilizers:
These fertilizers contain plant nutrients in their pure, mineral form, often referred to as “nutrient salts.” Many mineral fertilizers come with a coating that conceals the crystalline structure. The raw materials for these fertilizers are extracted from fossil deposits, with no direct connection to organic origins.
Organic Fertilizers:
In contrast, organic fertilizers present nutrients as part of an organic compound. These nutrients need to be released from organic structures by the plant or soil before they can be absorbed through the roots. This process involves various chemical reactions in the soil and, specifically, the root zone. While the availability of nutrients may take longer, organic materials in these fertilizers contribute to the formation of humus.
Organic-Mineral Fertilizers:
These fertilizers incorporate both mineral and organic components, sometimes featuring various compounds for a single nutrient. This blend aims to leverage the different speeds and properties of these compounds.
Moreover, these fertilizers come in liquid and solid forms. Liquid fertilizers contain nutrients in a dissolved state, providing immediate availability. Therefore, they are recommended in smaller doses but more frequently, approximately every two weeks depending on season. Some solid fertilizers can be dissolved in water, offering faster availability. Most NPK fertilizers for hobby gardening are commonly found in granular form, suitable for spreading.
Pros and Cons of NPK Mineral Fertilizers:
Pros:
- Contains the three main plant nutrients: Nitrogen, Phosphorus, and Potassium.
- Generally more cost-effective compared to organic or combined fertilizers.
- High nutrient content often results in immediate effectiveness.
Mineral nitrogen, in the form of ammonium, can slightly acidify the soil, while nitrate-form nitrogen tends to make it slightly more alkaline. Consider this when fertilizing pH-sensitive plants like Avocado and Rhododendrons.
Cons:
- Lack several essential nutrients required for plant growth.
- Prolonged use depletes soil reserves of other essential nutrients, leading to deficiencies over time.
- Mineral phosphorus is a finite resource, and conservation efforts are crucial.
- Artificial production of mineral nitrogen is very energy-intensive.
- Incorrect dosages can damage plants due to the rapid effectiveness.
- Washout of mineral nitrogen into groundwater during heavy rainfall can elevate nitrate levels.
- High nitrogen content, without stable organic matter, may lead to humus degradation, permanently compromising soil quality.
In essence, while mineral NPK fertilizers offer immediate visible results, it’s vital to recognize that plants require more than just Nitrogen, Phosphorus, and Potassium. Regularly supply your garden or potted plants with other macronutrients like Magnesium, Sulfur, and Calcium, along with trace elements, by alternating between different fertilizers.
Furthermore, incorporating organic matter is indispensable for maintaining soil fertility, making organic fertilizers a valuable choice as they address both objectives.
In conclusion, the thoughtful combination of various fertilizers, encompassing mineral and organic options, ensures a holistic approach to nourishing your plants and sustaining soil health.